Gulf Oil Spill Health Hazards

Many people will be exposed to chemicals in air, water, sand, soil, and food as a result of the oil spill in the Gulf of Mexico. It is important to understand the potential toxic effects and take appropriate protective actions to reduce exposure and harm.
This report describes the toxicity of chemicals in crude oil and in the dispersants currently being used in the Gulf area.

_______________________
Version 1.0 June 14, 2010
Copyright 2010 Sciencecorps, Lexington MA
Contents
General characteristics of dispersants
Micelles
Chemical ingredient issues
Current Products: Corexit 9527A and 9500(A)
Failure to warn and failure to fully disclose testing results
Communication with Congress
Chemicals and mixtures covered in this report include:
crude oil
benzene
propylene glycol
polypropylene glycol butyl ether
DSS
2-butoxyethanol
hydrotreated light petroleum distillates (Nopar 13 and kerosene)
The information provided in this report is based on medical scientific studies and documents cited throughout the report. Consult these for additional details. Updated reports will be available at: www.sciencecorps.org/crudeoilhazards.htm Additional information on chemical toxicity and the most up to date peer-reviewed medical scientific information is available on the National Library of Medicine's website: http://www.ncbi.nlm.nih.gov/sites/entrez?db=pubmed&TabCmd=Limits
This report should not be relied upon for diagnosis or medical treatment and does not provide specific medical guidance, which must be obtained from an individual's personal medical care provider.
Conflict of Interest Statement: The authors state that they have no conflicts of interest with respect to the information provided in this report and have no financial interests in the Gulf region or British Petroleum. This was a voluntary undertaking without funding, and is provided in the interest of public health.
Executive Summary
Chemicals in crude oil and dispersants can cause a wide range of health effects in people and wildlife, depending on the level of exposure and susceptibility. Crude oil has many highly toxic chemical ingredients that can damage every system in the body. Dispersant chemicals can affect many of the same organs. These include:
respiratory system nervous system, including the brain
liver reproductive/urogenital system
kidneys endocrine system
circulatory system gastrointestinal system
immune system sensory systems
musculoskeletal system hematopoietic system (blood forming)
skin and integumentary system disruption of normal metabolism
Damage to these systems can cause a wide range of diseases and conditions. Some may be immediately evident, and others can appear months or years later. The chemicals can impair normal growth and development through a variety of mechanisms, including endocrine disruption and direct fetal damage. They cause mutations that may lead to cancer and multi-generational birth defects. Some are known carcinogens, such as benzene (CDC, 1999).
Many of the chemicals in crude oil and the dispersants target the same organs in the human body, and this increases the risk and may increase the severity of harm. In addition, the dispersants currently used can increase the uptake (dose) of crude oil chemicals and movement of chemicals into critical organs.
Some people especially susceptible to harm are:
- those with pre-existing serious health conditions
- infants, children, and unborn babies
- pregnant women, especially those carrying multiple babies
- people working or living in conditions that impose health stresses, including exposures to other toxic chemicals
Individual responses depend on exposure and each individuals characteristics.
To fully understand and appropriately respond to chemical exposures that may result from crude oil and dispersants it is essential that additional information be provided by the federal government. This should include chemical concentrations of crude oil and dispersant chemicals and their breakdown and interactions byproducts in air, water, soil/sand, food, seafood and other media. Studies the government has on toxicity, persistence, and bioaccumulation should be made public. This information has not been adequately provided to the public or public health community. This severely limits the ability of people to make informed decisions and take appropriate protective action.
Personal and Public Protection
This report offers information to the health community and the public to improve access to relevant medical science, inform protective actions, and assist in identifying susceptible populations. The goal is to insure that people have sufficient information to make informed decisions, and that the public health community uses all reasonable means to inform and protect the public. A protective approach requires minimizing exposure to spill-related chemicals. Reducing exposure will invariably reduce the likelihood of harm.
Exposure to chemicals in crude oil and dispersants can occur through skin contact, inhalation of contaminated air or soil/sand, and ingestion of contaminated water or food. These can occur simultaneously.
In any work situation or community, there are locations where the likelihood of exposure to spill--related chemicals is highest. Recognition of those areas makes appropriate action more likely. When elevated exposure is likely due to air, water, food or other contamination, public advisories provide the best opportunity for protective actions by members of the public, and those charged with protecting them.
Some chemicals in crude oil and dispersants are volatile, moving into air easily.
Elevated levels of benzene, toluene, sulfur dioxide, and other toxic chemicals have been reported in coastal communities some distance from the spill. These reports from US EPA and other sources indicate increased airborne exposures of the general population occurred in these areas. Susceptible members of the public require notice when exposure may occur (e.g., when contaminated air masses move inland) so they can take protective actions, including moving to safer locations, using respirators, and other strategies.
In some cases, volatile organic chemicals (VOCs) released from crude oil can be detectable by smell. But not all hazardous airborne chemicals have a detectable odor. The absence of oil odors does not mean that there are no crude oil chemicals in the air.
Some information on the locations and amounts of chemicals is at: http://www.epa.gov/bpspill/index.html Unfortunately, the information is very limited and not readily accessible, as discussed below.
There have been some reassurances that crude oil reaching the shore is all "weathered". These statements are contradicted in many locations where shoreline oil is not weathered. When possible, obtain accurate local information from an objective source with the means to evaluate the oil's composition. Unweathered crude oil contains many VOCs and as it weathers, these chemicals are slowly released.
Claims that exposures to weathered crude oil do not pose health hazards are incorrect. Weathered oil is less toxic than unweathered crude oil with respect to the presence of VOCs and may have different properties with respect to skin absorption, depending on the degree of weathering.
Health and Safety Guidance from OSHA
There is considerable concern about workers who have become ill while cleaning up the oil spill. It is critical that people who work with or around crude oil wear appropriate personal protective equipment such as gloves, masks, respirators, and water repellant clothing, to minimize exposure. The necessary equipment will depend on the kind of exposure that can occur (dermal, inhalation, ingestion).
OSHA provides some guidance on worker health and safety that is relevant to marine oil spill exposures, and emphasizes the need for extensive training: "Training Marine Oil Spill Response Workers under OSHA's Hazardous Waste Operations and Emergency Response Standard". The following is important, though has been often ignored:
"...there are specific training requirements for all engaged in cleanup that range from a few hours to many days, in order to adequately prepare workers to deal with hazardous chemicals and preserve their health and safety..." (http://www.osha.gov/Publications/3172/3172.html )
This website includes a table of chemicals likely to be encountered in the oil, but does not include chemicals in dispersants. In addition, OSHA has updated other areas of their website and new information can usually be accessed through the main federal BP oil spill page.
Health and Safety Guidance from US EPA
The US EPA website that lists dispersant ingredients provides the following advice to workers:
"People working with dispersants are strongly advised to
- use a half face filter mask or
- an air-supplied breathing apparatus to protect their noses, throats & lungs,
- they should wear
nitrile or PVC gloves,
coveralls, boots, and
chemical splash goggles
to keep dispersants off skin and out of their eyes. "
Source: http://www.epa.gov/bpspill/dispersants.html Reformatted for clarity
Protective strategies should be employed by all people who may encounter crude oil or dispersants through direct contact, inhalation, ingestion, or contact with contaminated materials. Exposure should be minimized to the degree possible.
Whether an individual experiences health problems, and the severity and nature of those problems will depend on the combination and dose of chemicals they are exposed to, and their individual susceptibility. Populations that are especially susceptible are discussed below.
Ecology
This report focuses on human health effects, but most of the information is relevant to marine mammals, and some is useful for birds and fish. If you have expertise in ecotoxicology that may be relevant to public health, please share it with the public health community.
In general, the toxic effects in people that are caused by chemical exposures are similar to those that occur in mammals. Variations do exist, but the shared features in our anatomy and physiology provide a plausible scientific foundation for considering that toxic effects in people are likely to occur in mammals as well. Liver and kidney damage, reproductive damage, and other often observed effects are shared by many mammals.
Birds, fish, and other animals differ more in their anatomy and physiology, and so their responses to exposure to toxic chemicals are often less similar to humans than what occurs in mammals. However, some of the most fundamental and susceptible aspects of all vertebrate animals are shared in common - basic cell structure, DNA vulnerability, the critical nature of homeostasis, regulation of growth and development through nutrition and hormones, and other shared characteristics.
Chemicals that cause a wide range of toxic effects in people, as discussed in this report, are likely to have toxic effects on wildlife, pets, and agricultural animals. For more detailed information on this, consult a veterinarian, marine or avian biology specialist, the Veterinary Poison Control Center, or other specialists in this field.
Ecology is not restricted to animals, and the contamination and impacts of oil and dispersants on ecosystems is of concern to all people. It is beyond the scope of this report to speak to that issue. However, it is unquestionably true that the health of the ecosystems in which we live have a direct and substantial effect on the health of local communities. So it is important to be aware of any impacts that occur on local ecology, including surface and ground water supplies, as a result of the Gulf oil spill chemicals. Protecting local ecosystems insures the health of the environment and all of its residents.
Crude Oil Health Hazards
Crude oil contains hundreds of chemicals, many of them known to be toxic to people. Crude oil chemicals contain hydrogen and carbon (e.g., simple straight chain paraffins, aromatic ring structures, naphthenes), and some also contain sulfur, nitrogen, heavy metals, oxygen, and other elements compounds.
A list of common chemicals in crude oil is listed in Table D-1 of the U.S. Centers for Disease Control "Toxicological Profile for Petroleum Hydrocarbons"at:http://www.atsdr.cdc.gov/ToxProfiles/tp123.pdf (CDC, 1999).
Crude oil composition varies slightly by its source, but its toxic properties are fairly consistent. Chemicals such as benzene and polycyclic aromatic hydrocarbons (PAHs) are very toxic components of crude oil and of high concern. These and many other chemicals in crude oil are volatile, moving from the oil into the air. Once airborne, they can blow over the ocean for miles, reaching communities far from the spill. They may be noticed as petroleum odors. Consequently, both those working on the spill and people who are far from it can be exposed to crude oil chemicals in air.
One page summaries that can be used as handouts or small posters are available at the following webpages:
For the general Public: www.sciencecorps.org/crudeoilhazards-public.pdf
For workers: www.sciencecorps.org/crudeoilhazards-workers.pdf
Exposure
As noted above, exposure can occur through skin contact, inhalation of contaminated air or soil, and ingestion of contaminated water or food. Exposure may result in localized toxicity (e.g., irritation of the skin following contact), but many health effects are caused by systemic distribution of chemicals from crude oil, because ingredients can move throughout the body. Exposure varies based on the duration and concentrations in the contaminated material (air, water, soil, fish, etc). Differences in exposure will occur based on location, work and personal activities, age, diet, use of protective equipment, and other factors.
Concurrent exposure to other toxic chemicals at work and home must be considered when evaluating the potential for toxic effects to result from exposure to crude oil chemicals. Exposure to a combination of toxic chemicals, especially if they can damage the same organs in the body, increases the potential for health effects.
Detailed technical information is available on estimating the amount of exposure that people will have under various conditions (e.g., contaminated air inhalation volume) and on evaluations of children's unique exposure conditions: :
Adults (1997): http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=12464&CFID=7680523&CFTOKEN=45990564&jsessionid=2830d089441a1b1f5a60617f6564a6e531a3
Children (2003)
These documents make it very clear that exposure can vary considerably from one person to the next, and that children's exposure is often much higher, in relation to their body weight. What that means is that they may have a much higher dose of toxic chemicals than adults, who are in the same location.
Basic Physiological Effects
Crude oil is a complex mixture of chemicals that have varying abilities to be absorbed into the body through the skin, lungs, and during digestion of food and water. Most components of crude oil enter the bloodstream rapidly when they are inhaled or swallowed. Crude oil contains chemicals that rapidly penetrate the skin and move through cell walls. They can damage cell structures, including DNA, and alter the function of the cells and the organs.
A detailed discussion of the uptake, distribution, toxic effects, and elimination of chemicals in crude oil is summarized in the 1999 CDC publication at: http://www.atsdr.cdc.gov/ToxProfiles/tp123.pdf
One of the chemicals in crude oil that is of highest concern is benzene, because it has long been known to cause rapid toxic effects, and it is carcinogenic and mutagenic. A review the toxic effects and other characteristics of benzene is available at: http://www.atsdr.cdc.gov/toxprofiles/tp3.pdf. Benzene health effects are discussed under "chronic exposure hazards" below.
Toxic Effects: Systems Affected
Crude oil's toxic ingredients can damage every system in the body:
respiratory system nervous system, including the brain
liver reproductive/urogenital system
kidneys endocrine system
circulatory system gastrointestinal system
immune system sensory systems
musculoskeletal system hematopoietic system (blood forming)
skin and integumentary system metabolism
Damaging or altering these systems causes a wide range of diseases and conditions. In addition, interference with normal growth and development through endocrine disruption and direct damage to fetal tissue is caused by many crude oil ingredients (CDC, 1999). DNA damage can cause cancer and multi-generational birth defects (www.epa.gov).
"Acute" exposures generally refer to high exposure levels (e.g., in air, water, food) for a brief time. These kind of exposures are usually of greatest concern in the short-term, and may be the only effects described for chemicals that are not well-studied. The ability of crude oil's chemical ingredients to rapidly penetrate the skin and circulate through the body can result in rapid onset of symptoms and serious health effects
Crude oil contains many chemicals that can irritate the skin and mucous membranes on contact. Irritant effects can range from slight reddening to burning, swelling (edema), pain, and permanent skin damage. Commonly reported effects of acute exposure to crude oil through inhalation or ingestion include difficulty breathing, headaches, dizziness, nausea, confusion, and other central nervous system effects. These are more likely to be noticed than potentially more serious effects that don't have obvious signs and symptoms: lung, liver and kidney damage, infertility, immune system suppression, disruption of hormone levels, blood disorders, mutations, and cancer.
Benzene in the crude oil can cause a variety of specific effects described in the recent CDC summary of benzene toxicity: ventricular fibrillation, congestive gastritis, toxic gastritis, pyloric stenosis, myalgia, kidney damage, skin irritation and burns, swelling and edema, vascular congestion in the brain, and lethal central nervous system depression (http://www.atsdr.cdc.gov/toxprofiles/tp3.pdf ). Other health effects of longer term exposure to benzene are listed below.
In susceptible individuals such as children and those with health problems, moderate or low level exposures can cause effects usually associated with high exposures.
Due to the presence of chemicals in crude oil that are known to cause cancer in humans, and the fact that some of these chemicals can cause DNA damage and mutations, there is no completely safe level of exposure to many crude oil ingredients. While our bodies have mechanisms to repair damage, that does not always occur. US EPA's cancer risk assessment guidance documents discuss the scientific foundation for this, and clarify that any exposure to a mutagenic carcinogen (e.g., benzene) imposes some degree of risk (http://www.epa.gov/riskassessment/guidance.htm) Reducing exposure reduces cancer risks and the potential for other types of harm.
Chronic Exposure Hazards
Chronic exposure involves more than a few brief exposures to a chemical. Usually this occurs at relatively low levels. This type of exposure to crude oil should be avoided, if at all possible, because the potential for serious health damage is substantial. Chronic health effects are typically evaluated for specific crude oil components (see CDC, 1999), and vary from cancer to permanent neurological damage. They cover a range of diseases affecting all the organ systems listed above.
Acute effects do not fully predict the likelihood or severity of effects that may occur with longer-term low level exposures. That means that people who may not experience immediate effects such as headaches, tiredness, dizziness, nausea, or respiratory distress may still be at risk for health consequences.
Benzene is one of many chemicals for which very low exposure limits have been set by the federal government regarding worker exposure and exposures of the general public through drinking water and other means. This was done to minimize the potential for harm. In fact, US EPA's Maximum Contaminant Level Goal (MCLG) for benzene and other similar carcinogenic chemicals in drinking water is zero. This goal is set because any amount of exposure can pose a risk.
As an important component of crude oil, benzene is discussed below. Benzene is volatile and most of it may leave the crude oil after the oil is weathered. However, there are other chemicals that are similarly toxic that are not volatile. While benzene is discussed below, the absence of benzene from weathered crude oil does not mean that it is safe.
A 2007 CDC review of benzene toxicity concluded that there is substantial human evidence that benzene causes leukemia. They also report aplastic anemia (a precursor of leukemia), chromosomal abnormalities in lymphocytes and bone marrow cells, damage to the immune system and abnormal development of blood cells. When blood cells are deficient, this can cause other serious medical conditions, including infection due to a lack of leukocytes and increased cardiac stress due to a lack of erythrocytes. Long term low level oral and inhalation exposures have also caused peripheral nervous system abnormalities, distal neuropathy, difficulty sleeping, and memory loss (http://www.atsdr.cdc.gov/toxprofiles/tp3.pdf )
The International Agency for Research on Cancer (IARC), US EPA, and other entities have all agreed that benzene is a human carcinogen and can cause leukemia. Under these circumstances, EPA usually estimates a level of exposure that they consider to have "minimal" risk (e.g., one in one million chance). In the past, US EPA used the scientific studies of leukemia to calculate the level of benzene in air that they believed was likely to result in an increased risk of no greater than one in one million risk over a
lifetime to be 0.13 - 0. 45 µg/m3 which is equivalent to 0.04 to 0.14 ppb (see
Risk assessment guidance from US EPA that was finalized in 2005 now uses an adjustement to account for the much greater sensitivity of children to carcinogens. The "early life exposure" adjustement factor recommended by US EPA is usually approximately equal to three. Making that adjustment to the estimated values above results in an exposure level of:
0.01 ppb - 0.05 ppb is the benzene level in air that is expected to result in a cancer risk no greater than one in one million
It is important to keep in mind that this is for benzene only, and doesn't take into account other chemicals from the oil spill. In addition, levels in air have already been quite high in some locations following the spill. In order to minimize cancer risk, it is important to minimize future exposures.
Safety can't be determined by smell. Some people believe that if they don't smell the odor of oil, the air does not have any chemicals from the oil and is safe . However, that is not true. The odor threshold for benzene (the minimum amount of a chemical in air that people can smell) is approximately 1,500 ppb (US EPA, 2002). This is more than 10,000 times the level of 0.01 ppb that is listed above.
Animal study information is also available to assess the kinds of health effects that benzene may cause. Animal species are chosen for use in studies because they share important characteristics with people so that they are useful in predicting human responses. These studies found that inhalation exposure caused cardiovascular and liver abnormalities, depressed electrical activity in the brain, and other neurological abnormalities.
Oral exposure studies in animals resulted in endometrial polyps and ovarian lesions in females exposed for two years, and preputial gland lesions (reproductive system abnormalities ) in males. There is also some evidence that benzene can cause harm to the fetus, and reduce fertility (http://www.atsdr.cdc.gov/toxprofiles/tp3.pdf ).
Susceptible Subgroups
Children are vulnerable to toxic chemicals in crude oil that disrupt normal growth and development. Their brains are highly susceptible to many neurotoxic ingredients. Endocrine disruptors in crude oil can cause abnormal growth, infertility, and other health conditions. Children's exposures may be higher than adults and can include contaminated soil or sand. Newborns are especially vulnerable due to incompletely formed immune and detoxification systems.
Many people with medical conditions are more susceptible to crude oil toxicity because chemical ingredients can damage organ systems that are already impaired. Specific susceptibilities depend on the medical condition (e.g., inhalation poses risks for those with asthma and other respiratory conditions). Since chemicals in crude oil are able to damage most organ systems in the body, it is likely that most people with serious health conditions will be at elevated risk of harm if they are exposed to crude oil. Exposure to toxic chemicals can alter the actions of some medications, causing increased or decreased potency. A medical care provider and/or pharmacist can provide information on this.
People taking medications that reduce their detoxification ability, and those taking acetaminophen, aspirin, haloperidol, who have nutritional deficiencies or who concurrently drink alcohol may be more susceptible. Some inherited enzyme deficiencies also increase susceptibility (listed in CDC, 1999).
People exposed to other toxic chemicals at work or home may be at higher risk.
Pregnancy places increased stress on many organ systems, including the liver, kidneys, and cardiovascular system. Chemicals in crude oil that are toxic to these same systems can pose serious health risks. Pregnancy also requires a careful balance of hormones to maintain a health pregnancy and healthy baby. Endocrine disruptors in crude oil can jeopardize the hormone balance and some alterations can have serious consequences for cognitive development (e.g., reduced thyroid function). Women who are carrying multiple babies are at higher risk for kidney damage due to the greater physiological stress this causes.
The developing fetus is susceptible to the toxic effects of many chemicals in crude oil. Many cause mutations, endocrine disruption, skeletal deformities, hormone disruption, neuorocognitive damage, and other types of damage. Different aspects development are at high risk during different months of gestation. Most chemicals circulating in the mother can pass through the placenta and reach a baby. Considerable care should be taken to obtain information regarding local contamination and to consult with a prenatal heatlh care specialist regarding appropriate actions to protect both the mother and baby.
Sources
CDC, 2007: http://www.atsdr.cdc.gov/toxprofiles/tp3.pdf
US EPA, 2002 on benzene: http://www.epa.gov/ttn/atw/hlthef/benzene.html
OSHA, 2010: http://www.osha.gov/Publications/3172/3172.html
NLM: http://sis.nlm.nih.gov/dimrc/oilspills.html - very limited information on human health
The National Toxicology Program (NIEHS-NIH) provides information on carcinogenic crude oil ingredients (e.g., benzene) & limited information on reproductive hazards http://ntp.niehs.nih.gov/
California's EPA provides a list of chemicals know to cause cancer and/or reproductive harm: http://www.oehha.org/prop65/prop65_list/files/P65single040210.pdf
US EPA's Exposure Factors Handbook, 1997 (not yet updated): http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=12464&CFID=7680523&CFTOKEN=45990564&jsessionid=2830d089441a1b1f5a60617f6564a6e531a3
US EPA's Exposure Factors Handbook for Children (2003)
Children's Health - International pediatric consensus statement regarding children's susceptibility to toxic chemicals: http://www3.interscience.wiley.com/cgi-bin/fulltext/119425377/HTMLSTART This contains a link to 120 scientific papers presented at the Conference on Children's Susceptibility to Environmental Hazards.
Federal focus on children's environmental health including policies designed to protect children: http://yosemite.epa.gov/ochp/ochpweb.nsf/content/homepage.htm
Many products are used on oil spills, including dispersants, surface washing and collecting agents, and bioremediation agents. This report discusses potential health effects of the dispersants used on the BP Gulf of Mexico spill through June 14, 2010.
Crude oil and dispersants contain chemicals that are hazardous individually and in combination. The likelihood of harm depends on dose and individual susceptibility.
Products discussed in this section include Dispersit 9527 and 9500, with the following chemical ingredients:
propylene glycol
polypropylene glycol butyl ether
dioctyl sodium sulfosuccinate (DSS)
2-butoxyethanol (2-BE)
hydrotreated light petroleum distillates (Norpar-13 and kerosene)
General Characteristics of Dispersants
Oil spill dispersants have many actions, including acting as solvents that can mix with the crude oil mass, and move through it. In 2005, the National Research Council (NRC) published a book in 2005 on oil spill dispersants available at: http://www.nap.edu/openbook.php?record_id=11283 (full text online). It provides detailed information on the use of dispersants, chemical ingredients, studies conducted to determine efficacy, and refers to Corexit products extensively. It also contains extensive references and links to other publications.
The 2005 NRC report cites studies indicating that dispersants can increase the uptake of oil by organisms. This is scientifically plausible when you consider that cells of all animals, including humans, have walls made of lipids. Lipids are fats, very similar to simple oil hydrocarbons that are in crude oil. Detergents, surfactants, and solvents make it easier to move through the oil.
The properties that facilitate dispersants moving into an oil spill to disperse it, also make it easier for them to move through cell walls, skin barriers, and many other protective coatings we rely on to protect vital organs, underlying layers of skin, the surfaces of our eyes and other structures. In discussions of the potential health effects of individual chemical ingredients in dispersants below, evidence is provided regarding dispersant chemicals' ability to increase chemical uptake into people.
The dispersants Corexit 9500 and 9527A have been used, according to the federal Gulf spill website at http://www.deepwaterhorizonresponse.com/go/site/2931/ A list of EPA-approved dispersants, with limited ingredient and chemical property information. is available at "National Oil and Hazardous Substances Pollution Contingency Plan Product Schedule" athttp://www.epa.gov/emergencies/docs/oil/ncp/notebook.pdf (dated March 2010).
Micelles: Crude Oil & Dispersants in a Bubble
To understand the potential health effects of dispersants, it is necessary to understand that exposure may involve a specialized structure, a micelle, that is described below. This structure impacts the behavior and toxicity of chemicals in oil and in the dispersants. The characteristics of micelles are important when considering how to protect the public and ecosystems.
Corexit dispersants are designed to form micelles, small bubble-like structures, to envelop crude oil droplets. The 2005 NRC book on dispersants illustrates micelles formed by dispersants interaction with oil and the processes that lead to their formation (image below from page 55).

Micelles have a portion of dispersant chemical on the outside, in contact with ocean water. Another portion is on the inside where the oil is located. Micelles range in size, but most are very small, in the 10 micrometer (um) range according the NRC report: http://www.nap.edu/openbook.php?record_id=11283&page=57 Images and text in the this report show how dispersants act to break up oil masses into small dispersant-coated oil droplets.
The combination of detergent and hydrocarbons ingredients in dispersants with chemicals in crude oil is especially hazardous if someone inhales contaminated water spray. The dispersant-oil complex in micelles can coat lung surfaces causing lipoid pneumonia, hypersensitivity pneumonitis, asthma and other serious health problems.
Headaches and chest tightness may result from respiratory problems, which is why evaluations of lung function are a part of health evaluations for exposure to these chemicals, even if respiratory symptoms aren't immediately obvious. Failure to recognize this can have serious consequences.
Chemicals in dispersants, including surfactants, detergents and others, can damage the lungs of mammals and birds. The gills of fish can become covered with a film that prevents them from breathing. Crude oil chemical ingredients and dispersants both cause damage independently and in combination.
For additional information on micelles and the dispersant application process see a presentation by a scientist with Corexit's former manufacturer http://www.chemie.uni- regensburg.de/Physikalische_Chemie/Kunz/student/Uebung_Formulierung/Clark_presentation.pdf
Characteristics of micelles may make it very difficult to identify oil in the water, since the oil-filled micelles do not appear as the standard oil sheen on the water. They may be below the surface and not be detectible by sight or smell. This can create an invisible hazard for the public.
Chemical Ingredient Issues
US EPA provided a list of two Corexit products' ingredients on June 8th that is provided at the end of this report. (EPA : http://www.epa.gov/bpspill/dispersants.html )
Products used on spills typically have multiple chemical ingredients. Companies are not required to list all ingredients in their products, or to provide detailed information on those that they do list. They can claim ingredients are "proprietary" to avoid disclosure.
For example, the MSDS for Corexit products lists "organic sulfonic acid salts" as an ingredient. It does not specify the organic component. There are many potential organic components. Without specific information, it isn't possible to be certain of short or long-term human health hazards or ecological effects. Although US EPA provided some additional information on the ingredients, they did not provide specific details for all of them, as discussed below.
Ingredients in a product may be listed as a group rather than a single chemical. For example, the group "petroleum distillates, hydrotreated light" is listed on the MSDS for Corexit 9500. There are many chemicals within this group. While this may be customary, without a precise description, we cannot fully assess the potential human or ecological effects.
When specific chemical information is lacking, it may be possible to make a reasonable scientific estimate of the approximate chemical structure. Estimates are provided here for the sole purpose of evaluating potential health hazards and providing the public and the health community with essential information.
Susceptible Subgroups
Exposure to dispersants is likely to occur together with exposure to crude oil. The toxicity of crude oil is wide ranging and so the discussion of susceptible subgroups above is sufficient to address the major groups of concern. Some additional susceptibility issues are discussed for specific dispersant chemical ingredients addressed below.
Current Dispersants: Corexit 9527A and Corexit 9500
Two dispersant products have been used on the Gulf spill according to the federal spill website at http://www.deepwaterhorizonresponse.com/go/site/2931/ They are discussed below, with a focus n the specific chemical ingredients known to date. These products share most ingredients in common.
Unfortunately, information on the breakdown and interaction by products of these chemical in water and crude oil have not been disclosed. These additional chemicals may have similar toxic properties, or may vary from the original chemicals that were applied to crude oil. Consequently, while this report contains information on crude oil and most known dispersant ingredients, it is not possible to determine the degree to which it captures the range of toxic effects that may occur.
We strongly encourage the federal government to rapidly disclose information regarding the actual array of chemicals that are forming and to which people are being exposed.
Sources of Information on Chemical Ingredients
Limited information on safety is provided the Corexit manufacturer (Nalco) in Material Safety Data Sheets (MSDS) for each product (links below).
The US EPA's NCP product guide also provides some information at: http://www.epa.gov/emergencies/docs/oil/ncp/notebook.pdf
Additional information on product use and ingredients was obtained from the 2005 NRC publication on oil spill dispersants referred to above (http://www.nap.edu/openbook.php?record_id=11283).
A 1996 journal article titled "Comparison of Acute Aquatic Effects of the Oil Dispersant Corexit 9500 with Those of Other Corexit Series Dispersants" provides information on the composition and toxicity (note - information is 14 years old). (http://www.envtox.ucdavis.edu/GraniteCanyon/MS%201996%20EES%20acute%20tox.pdf
On June 8, 2010, US EPA provided additional, but not complete information on dispersant ingredients ( http://www.epa.gov/bpspill/dispersants.html#chemicals)
Corexit 9527A
This product was used in the Gulf until supplies ran out in May 2010 and was used extensively on the Exxon Valdez spill in Alaska. Literature related to this dispersant and the Alaska experience is relevant. A review of that information was compiled in 2002 : http://www.pwsrcac.org/docs/d0002700.pdf
A Material Safety Data Sheet (MSDS) written by the manufacture, Nalco, is available at: http://www.deepwaterhorizonresponse.com/posted/2931/Master_EC9527A_MSDS.539295.pdf.
The NCP product guide from EPA provides information on this product on page 1: http://www.epa.gov/emergencies/docs/oil/ncp/notebook.pdf
According to these sources, the Corexit 9527A ingredients are:
2-butoxyethanol
propylene glycol
organic sulfonic acid salts
Polypropylene glycol butyl ether was listed by US EPA, June 8, 2010 as an ingredient of dispersant, but they did not indicate if one or both products contained it. (Seehttp://www.epa.gov/bpspill/dispersants.html#chemicals)
NRC 2005 provides additional information on ingredients in Corexit 9527A and 9500, stating:
"Both products contain a mixture of nonionic (48 percent) and anionic (35 percent) surfactants. The major nonionic surfactants include ethoxylated sorbitan mono- and trioleates and sorbitan monooleate; the major ionic surfactant is sodium dioctylsulfosuccinate." (pages 55-56)
They list the following surfactants on page 55:
sodium dioctyl sulfonosuccinate (DSS) - ionic
Span-80 (CAS # 1338-43-8) sorbitan monooleate - nonionic
Tween-80 (Cas# 9005-65-6) ethoxylated (E20) sorbitan monooleate - nonionic
Health effects information is provided below on the ionic surfactant, DSS. The nonionic surfactants are not clearly specified because EPA lists "derivatives", which may have various chemical structures. Information on the nonionic surfactants will be provided if more specific information becomes is available.
Propylene glycol This ingredient is in both Corexit products.
Propylene glycol is a clear liquid with almost no odor and a sweet taste. It can be made from petroleum or biomaterials. Propylene glycol is in the general family of glycol ethers and is used as feedstock for chemical formulations, as a solvent, lubricant, veterinary medication, antimicrobial, preservative, stabilizer, and as an emollient in cosmetics and pharmaceutical creams. Propylene glycol has solvent properties and is used as a solvent in many applications. It is an alternative to ethylene glycol in some applications, including antifreeze. The FDA designated propylene glycol as "generally recognized as safe". However, concerns have been raised about health hazards. It has been licensed for sale as a pesticide. It is produced by Dow, Huntsman International, Arch Chemicals, and Lyondell Chemical in the United States and sold in multiple grades, with the largest reported use being for polyester resins. The 8 hour time weighted average workplace exposure limit is 50 ppm.
The information in this section was obtained from the Hazardous Substances Data Bank, the CDC-ATSDR 1997 Toxicological Profile and heir addendum released in 2008, unless otherwise noted. Links to these resources are provided below.
Propylene glycol is a mild irritant, and its role as an allergic dermal sensitizer is not resolved. Exposure to high levels of propylene glycol and mists containing this chemical can cause eye, nose, throat, and lung irritation, based on observations of effects caused in people. Some people are allergic to this chemical, and those with eczema may be at higher risk. Erythema, edema, induration, and other skin problems have been reported. Dermal effects have occurred following oral exposures in addition to direct dermal contact. Skin sensitization can occur following any route of exposure (after an initial exposure, subsequent exposures may cause a more severe reaction). According to CDC 2008, propylene glycol is a well known allergen.
Dermal exposures have caused serious health problems in some individuals, including cardiorespiratory arrest in an infant exposed to propylene glycol in a medication. Infants, especially premature infants, have an incompletely formed dermal barrier, making their skin more susceptible to damage, and more easily penetrated by chemicals that come in contact with their skin. This is relevant if there is any anticipated contamination of water or other media that may contact the skin of infants.
In individuals that tested positive in a propylene glycol patch test, adverse neurological reactions occurred. High levels of exposure have also caused neurotoxic effects in people, primarily central nervous system depression, including narcosis. In a highly exposed child, this occurred with metabolic acidosis. In general, this chemical has a mild anesthetic effects.
Animal studies have also found neurotoxic effects of propylene glycol, including incoordination, poor body/limb tone, decreased respiration, and lethargy. This was hypothesized to be linked to metabolic disruption (D-lactate). In humans and many species of animals, propylene glycol is metabolized in the liver via alcohol dehydrogenase producing lactaldehyde and then via aldehyde dehydrogenase to lactate. The resulting lactic and pyruvic acids can cause metabolic acidosis and related sequelae. Repeated doses have caused hypotension and cardiac arrhythmias, rapid breathing, and other cardiovascular symptoms. At high exposures, kidney damage has been observed in some species.
It is rapidly distributed throughout the body via fluids and in studies of distribution, the concentrations in cerebrospinal fluid were very close to those found in serum. Inhalation resulted in a minimal dose to the lungs. Clearance occurs rapidly, on the order of a few hours. There is substantial variation between individuals in the metabolism and clearance of propylene glycol and consequently in the effects observed.
Case reports raise questions regarding the assertion of low toxicity of propylene glycol and the elevated susceptibility of infants and children. A severe reaction was observed in a premature infant (coma) following application of dressings for burns. Ingestion of a small quantity in hair gel by a two year old resulted in marked acidosis and CNS depression and in another two year old, chewing on disposable cleaning towels containing propylene glycol resulted in severe CNS depression. Tests were done in each case to confirm serum propylene glycol levels.
Animal studies have yielded similar toxicity characteristics to observations in humans with a few additional toxicity manifestations. These studies employ animals that are specifically chosen to predict what may happen in humans. Consequently, it is important to pay attention to the results of those studies, as they may occur in people.
The blood forming system (hematopoietic) has been the subject of considerable study because of the wide range of problems observed in animal studies. The following pathological changes were observed in animal studies with exposures of different lengths: decrease in white blood cells and lymphocytes, severe cell fragility, adrenocortical hemorrhage, abnormally structured blood cells, reduced hemoglobin content, reduced packed cell volume were reported, cell membrane ruptures, rough cell surfaces, increased cell adherence. Changes in bone marrow, where blood cells are formed, were also reported. Dogs in a 2 year feeding study had increased rates of erythrocyte hemolysis and a lowered Hb content (reversible when exposure ceased).
There were differences in the pathology between species, though a breakdown of red blood cells was common to many. These types of effects can have very serious implications for human health across many systems because blood cells are essential to carry oxygen to the brain, heart, muscles and other organs. Impacts on cell surface properties could have implications for human development, especially in the vascular system.
Orally exposed male rats had elevated liver total cholesterol levels.
A multigeneration reproductive study in rats reported lowered food consumption, slower growth, later pregnancy, and smaller litters. At the highest dose tested, the females did not breed or raise their young normally. Oral exposure produced females "unable to bring their young to weaning". Propylene glycol caused decreased fertility and ovarian weight. Their pups had decreased body weight, pup survival was reduced, and there were fewer pups in each litter. The also had delayed puberty and abnormal cells in their livers and thymus. This occurred in both the first and second generation following maternal exposure to propylene glycol.
Developmental damage was also observed in a study of animal reproductive cells. Cell membranes were damaged, and the pH was altered, causing a decrease in the development of the embryo.
In a study designed to determine whether propylene glycol may play a role in aneuploidy (chromosomal abnormality), mouse oocytes (reproductive cells) were studied following exposure of live mice. Increases in the proportion of oocytes with abnormal genetic processes (aberrant centromere separation - PCS) and in aneuploidy were observed. The authors concluded that propylene glycol-induced PCS predisposes zygotes (early stage offspring) to aneuploidy. In male mice the chemical caused chromosomal aberrations in their spermatocytes.
Information on propylene glycol toxicity remains incomplete. Genotoxicity, endocrine disruption, neurotoxic, cardiotoxic, reproductive, and other effects have not been fully evaluated.
It is unlikely that most people will be exposed to very high concentrations of propylene glycol from the dispersants. However, this may be a problem for those working on the spill cleanup, and there may be some people who are especially susceptible to this, due to genetics or ongoing health problems.
Summary
Propylene glycol does not have a high apparent acute toxicity for everyone, but some people are very sensitive to it. The multiple systems where damage or disruption has been reported in animal studies and in high exposure incidents in people suggest that care must be taken with this chemical. CDC's "minimal risk level" is 9 parts per billion in air for moderate term exposures (15 - 364 days). This low MRL suggests that precautions against exposure are appropriate.
Systems most affected include the blood forming, reproductive. central nervous system, and skin (in some people). A recent study showing maternal exposure caused delayed puberty and abnormal liver and thymus cells in offspring raises concerns regarding prenatal exposures. A protective approach requires this information be considered for pregnant women. Additional research should rapidly explore this issue.
Dispersants issue. An important characteristic of propylene glycol is its ability to increase penetration of some chemicals through the skin (CDC, 2008, p8). This is beneficial for some medicines applied through the skin. But increased skin penetration of toxic chemicals that are in crude oil or the dispersants would increase the dose of those chemicals inside the body, thereby increasing their toxic effects.
Ecological effects - CDC 2008 contains a brief description of harm caused to an avian species. This is not directly relevant to humans, but is noteworthy for those working on avian toxicology.
Persistence - Biodegradation of propylene glycol relies on oxygen. In surface water that will likely occur within several days (CDC-ATDSR, 2008). However, in deep water where oxygen levels are lower, the process will take longer. In addition, because it relies on oxygen for degradation, propylene glycol exerts a biological oxygen demand (BOD) that reduces oxygen available to marine life. Depending on how long after application the dispersants reach shore or are otherwise accessible to people, the propylene glycol may have broken down, or may still be present in the water. It will likely be highly diluted unless it was just recently applied locally.
Sources:
CDC-ATSDR. 1997. Toxicological Profile on Propylene Glycol and Ethylene Glycol Atlanta, GA. http://www.atsdr.cdc.gov/toxprofiles/tp189-c2.pdf
Addendum for Propylene Glycol. Supplement to 1997 Toxicological Profile for Propylene Glycol and Ethylene Glycol. 2008. Atlanta, GA.
Note - The summary in this document is cited to Dow Chemical, a manufacturer of propylene glycol. A review of the entire report is recommended
Hazardous Substances Data Bank http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
Polypropylene glycol butyl ether
This chemical was specified June 8th by US EPA as an ingredient of Corexit 9527A and/or 9500A. However, the Nalco MSDS's that EPA provided on the same website do not list this, so we lack information on the percentage it comprises in the dispersants: (http://www.epa.gov/bpspill/dispersants.html#chemicals).
This chemical was not disclosed on the Nalco MSDS's previously available. It is not listed in the MSDSs that US EPA links to from their website as of June 8th, 2010:http://www.epa.gov/bpspill/dispersants.html#chemicals
Material Data Safety Sheet for Corexit 9500A (PDF) (11pp., 88 K, About PDF)
Material Data Safety Sheet for Corexit 9527A (PDF) (11 pp., 132 K, About PDF)
Since they are not listed, there is no indication of which Corexit they are present in, There is also no way to know what percentage of the product they comprise.
Limited health effects information is provided for this chemical because there is little readily available to scientists. However, extensive information should be available on this chemical because it apparently was and may still be a pesticide. Toxicology, persistence, and other study results are required for registration. That information should be provided on EPA's dispersant website. This chemical is also an ingredient in cosmetics, so toxicity information from the FDA's system should be provided.
PPGBE (an abbreviation used here for simplicity) is in the propylene glycol ethers family (per ToxNet, a National Library of Medicine website).
The chemical structure is as follows: 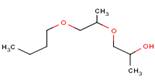

Source: Chem ID Plus at: http://chem.sis.nlm.nih.gov/chemidplus/jsp/common/ChemInfo.jsp?calledFrom=lite&type=names This has a more complex structure than propylene glycol, discussed above.
According to the US DHHS Household Products Database http://hpd.nlm.nih.gov/cgi-bin/household/search?queryx=29911-28-2&tbl=TblChemicals&prodcat=all ) PPGBE is used in paints, varnish, adhesives, bathroom cleaners, disinfectants, scum removers and other similar products.
The IUCLID data system, a compilation of toxicology and related information from the European Union, provides information on this chemical at: http://ecb.jrc.ec.europa.eu/IUCLID-DataSheets/29911282.pdf . Unfortunately, most study data was submitted by Dow Deutchland Inc and most studies relevant to human health are not listed as having been published in the peer reviewed scientific medical literature. IUCLID provides limited information on aquatic toxicity as well as some information relevant to human toxicity, The information below is from a summary of the IUCLID abstracts, unless otherwise noted.
In two week animal studies of exposure through inhalation the following effects were reported:
reduced weight gain,
porphyrin on the external nares (nose)
perineal soiling,
mild hemoconcentration,
decreased activity and lethargy,
increased mean liver weights,
histopathologic alterations in the liver (panlobular increased size of hepatocytes)
histopathologic alterations in nasal cavities (hyperplasia of the anterior respiratory epithelium, sometimes accompanied by suppurative inflammation, squamous metaplasia of the respiratory epithelium, or degeneration of the anterior olfactory epithelium)
lymphoid depletion of the thymus and/or spleen.
Oral feeding studies in animals exposed for 90 days found:
changes in clinical chemistry,
reduced body weight,
increased magnesium level in urine,
increased urea plasma levels.
A 90 day dermal exposure study in animals, using propylene glycol used as the control, found:
mild skin irritation,
reduced body weight,
a shift from lymphocytes to neutrophilic granulocytes in the differential white blood cell count,
increased relative liver weight.
PPGBE did not cause skin sensitization in human testing.
Genotoxicity information is unclear in the IUCLID system. Multiple positive results are reported. However, according to the record submitted by Dow, these may be attributable to problems with the studies.
Studies with the chemical radiolabeled to track where it went in the body found that there was migration into the bone marrow in study animals.
The above information is listed with the source "Dow Deutschland Inc". In most cases, the toxic effects were accompanied by statements that results were not toxicologically relevant or were due irrelevant factors. Some health effects were attributed to stress. Various other reasons were listed for disregarding results of multiple short and long-term studies.
Metabolites of this chemical excreted in urine include: propylene glycol n-butyl ether, dipropylene glycol, propylene, sulfate conjugate of the chemical, and the parent chemical.
No long term exposure studies were reported, which prevents any conclusions regarding the carcinogenic nature of this chemical. No multigeneration reproductive/developmental toxicity study was listed.
The following information is provided due to the very limited data available on PPGBE, and the lack of information free from conflicts of interest. PPGBE is a member of a much larger group of polypropylene glycols (PPGs), that has many members studied more extensively. Some PPGs are potent stimulators of the central nervous system (CNS) and can readily cause cardiac arrhythmias. Depending on their molecular size, which ranges widely, they are not generally skin irritants or skin sensitizers. It is not known whether PPGBE will elicit health damage similar to other PPGs, so this information is provided strictly as additional information that may be used to prompt new and more comprehensive studies of PPGBE.
Sources:
Hazardous substances Data Base (HSDB) http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
2-butoxyethanol (2-BE)
The scientific evidence on toxic effects of 2-BE was provided in 2007 to a city considering banning its use in cleaning products by city employees. The summary below is taken from that report on the medical scientific literature, available at: http://www.sciencecorps.org/KMB_2-butoxyethanol_toxicity.pdf. The report was prepared for a different use of 2-BE, and the dispersant's chemical mixture and formation of micelles containing oil must be considered when evaluating potential heath effects of 2-BE in the Gulf. Since there is a recent detailed report available for this chemical via link, only a brief summary is provided below.
Summary:
Based on scientific information available primarily from animal studies designed to predict human health effects, 2-BE may cause respiratory, blood cell, reproductive, and developmental damage.
There is strong evidence that 2-BE can damage the respiratory system, including causing asthma attacks and increasing the symptoms and severity of many other respiratory diseases (e.g., emphysema, chronic obstructive pulmonary disease, upper and lower respiratory infections). People with respiratory diseases should avoid exposure to air or water that may contain dispersants.
Red blood cells are also a target of 2-BE's toxic effects. The damage that it causes to these cells can lead to damage in other parts of the body that the cells travel to, including the liver, kidney, spleen, and other organs. People at risk include those with diseases or hereditary conditions involving blood (e.g., anemia, Sickle Cell Disease, liver or kidney disease). Women who are pregnant and therefore must produce red blood cells rapidly to support fetal growth and maintain their health are also a group of high concern.
The National Toxicology Program within the National Institutes of Health found some evidence that 2-BE causes cancer, based on many types of tumors caused by 2-BE in animal studies. There is also limited evidence that 2-BE can cause genetic damage (e.g., mutations), which can initiate cancer and developmental damage.
There is scientific evidence from animal studies that 2-BE exposure before birth may cause delayed bone formation and fetal death. Many chemicals in the glycol ether family are known to cause severe harm during development. Damage to the reproductive system was reported in study animals, including reduced testicular size.
There is not consensus regarding the potential for harm at very low exposure levels. A recent evaluation by California concluded that reproductive toxicity should be the basis for controlling short-term high level exposures. In the absence of definitive information on this important health impact, a protective approach is recommended. As you will see, there is reason to protect pregnant women, children, and some other susceptible populations from even low exposures.
Sources
California OEHHA. 1999. Determination of Acute Reference Exposure Levels for airborne Toxicants. Acute Toxicity Summary: Ethylene Glycol Monobutyl Ether. http://www.oehha.ca.gov/air/acute_rels/pdf/111762A.pdf
Centers for Disease Control (CDC-ATDSR). (1999). Toxicological Profile for Butoxyethanol and 2-Butoxyethanol Acetate. US Department of Health and Human Services. ATDSR-CDC, Atlanta, Georgia. http://www.atsdr.cdc.gov/toxprofiles/tp118.html
MSDS: 2-BE as a pure undiluted product, by Mallinckrodt Baker, Inc. Phillipsburg, NJ
New Jersey Hazardous Substances Fact Sheet for 2-BE that is available at: http://nj.gov/health/eoh/rtkweb/documents/fs/0275.pdf
Nazaroff, W., et al. 2006. Indoor Air Chemistry: Cleaning Agents, Ozone and Toxic Air Contaminants. CARB. Sacramento California. (full text free online)
NIOSH 1990. Criteria for a recommended standard: Occupational exposure to ethylene glycol monobutyl ether and ethylene glycol monobutyl ether acetate. Cincinnati, OH: NIOSH, NTIS No. PB91-173369. (full text free online)
National Toxicology Program (NTP). 1985. Ethylene glycol monobutyl ether (CAS #111-76-2): Reproduction and fertility assessment in CD-1 mice when administered in drinking water. NIEHS, NIH. RTP, NC. Abstract:http://ntp.niehs.nih.gov/go/14577
NTP evaluation: http://ntp.niehs.nih.gov/go/25759
NTP. 1989. Teratologic evaluation of ethylene glycol monobutyl ether (CAS # 111-76-2) administered to Fischer-344 Rats on either gestational days 9 through 11 or 11 through 13. Abstract: http://ntp.niehs.nih.gov/go/7776
NTP. 1993. Ethylene glycol ethers, 2-ethoxyethanol, 2-butoxyethanol administered in drinking water to F344/N rats and B6C3Fl mice. NTP toxicity report series 26. NIH Publication 93-3349 (full text free online)
NTP. 2000. NTP Technical Report on the Toxicology and Carcinogenesis Studies of 2-Butoxyethanol in F344/N rats & B6C3f1 mice. NTP TR 484 NTP, RTP, NC http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr484.pdf
Organic sulfonic acid salt This ingredient is in both Corexit products.
Preliminary information regarding physical properties, reference toxicants, and other characteristics specified in the MSDS provided by the manufacturer, and US EPA's NCP guide, and other sources listed above were reviewed by Dr. Alex Madonik, a consulting chemist with the Green Science Policy Institute (bio, http://www.greensciencepolicyinstitute.org/). Based on available information, he was determined that this chemical is likely to have a form that is common to other surfactants and detergents. Dioctyl sodium sulfosuccinate (DSS) is an organic sulfonic acid salt that is discussed as an ingredient of dispersants in the NRC (2005) publication on this topic. Dr. Madonik determined that this is a likely ingredient and the information below discusses this chemical. If additional information becomes available, we will provide it.
The following information is provided because it is likely that DSS or a similar chemical is in both Corexit products used in the Gulf. In general, structurally similar chemicals have similar toxicology, though that is not always the case. If additional information is provided by the manufacturer or US EPA and indicates a different chemical composition than indicated on their current website, the text below will be revised. The information below is provided as the best available approximation at this time, and is offered to assist the public and health professionals in considering protective strategies and potential health effects. As noted above, in the case of actual poisoning, the manufacturer is required to provide complete ingredient information to medical care providers.
With respect to the micelles described above, anionic surfactants are on the outside and enable mixing with water due to their polarity. The hydrocarbon chain, which is non-polar, is on the inside of the micelle, where the oil droplet is located. A sulfonic acid salt is a detergent, typically with a hydrocarbon chain and absent halogens. It may have other common uses, as is the case with DSS.
Dioctyl sodium sulfosuccinate (DSS)
Dioctyl sodium sulfosuccinate (DSS) is an organic sulfonic acid salt. There are many chemical and trade names for this chemical, and they can be searched at:http://chem.sis.nlm.nih.gov/chemidplus/ The Hazardous Substances Data Bank (HSDB) provides basic information on this chemical at: http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@rn+@rel+577-11-7 (last revised 2001) under the name: bis(2-ethylhexyl) sodium sulfosuccinate. The information below is taken from this source, unless otherwise noted.
DSS is used as a dispersing and emulsifying agent in dermatology preparations, as a good additive, in cosmetic applications, in industrial uses, as a pet shampoo to remove fleas, and as a pharmaceutical (laxative). Exposure can occur through dermal contact, inhalation, and ingestion.
DSS is irritating to the eyes and throat, there are reports of allergies, and it may cause aspirin's gastrointestinal effects (mucosal damage) to be increased. It is absorbed from the GI tract and excreted largely in the bile. Like many detergents, it has laxative effects. DSS can increase the uptake of mineral oil and phenophthalein, suggesting that it may also increase the uptake of other oils and chemicals. HSDB reports that the AMA Council on Drugs identified docusate salts as potentially being toxic to the liver. The HSDB describes DSS as "moderately toxic".
A study of the inhalation exposure of experimental animals (mammals) to DSS resulted in peribronchial and focal alveolar edema in three of the five subjects. This was believed to be caused by the action of DSS on the surfactant system of the lungs (see study abstract at: http://www.ncbi.nlm.nih.gov/pubmed/9053567 ) This is relevant to dispersants primarily if workers are exposed during application, when the concentration could be fairly high. It will likely be very dilute if it persists to the shoreline.
Information on this chemicals use as a pesticide and additional resources are is available at: http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC33310
Summary
If workers are exposed, it is not acutely toxic upon ingestion, but inhalation poses a risk of respiratory system damage, where lung function can be reduced. That is also true of many other ingredients in the dispersants, so the combination of respiratory toxins in terms of the total volume inhaled is relevant. Respiratory system damage may not be readily recognized and can result in central nervous system and cardiovascular problems. Consequently, a worker exposed to dispersants who is suffering from difficulty breathing, neurological, or cardiovascular difficulties should have a lung function test performed to determine if they have inhaled a dangerously high dose of detergents, surfactants, or other chemicals that were in their work environment while working on the oil spill. Since detergents may facilitate the uptake of oil, the toxicity of the detergent (e.g., DSS) and oil combination may be increased. Those with pre-existing respiratory conditions are at particular risk if this is inhaled.
It is unlikely that the general public will be exposed to substantial concentrations of DSS, but if that is the case, the information provided above is relevant.
Ecotoxicity - See the following source: http://www.pesticideinfo.org/List_AquireAll.jsp?Rec_Id=PC33310 Detergents are toxic to marine life if exposure causes the gills to be damaged or if respiration is otherwise reduced. Respiratory damage was noted following inhalation in the mammalian study cited above.
Persistence - In water, DSS is non-volatile and has a half life of approximately 6.7 years at a pH of 7 (water). I may be biodegraded rapidly. It does not appear to bioaccumulate, but can adsorb to sediment. It will exist primarily in the particulate phase in the ambient atmosphere and is a solid at room temperature.
Sources:
Journal Article: http://www.ncbi.nlm.nih.gov/pubmed/9053567
DSS as a pesticide product, with links: http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC33310
Corexit 9500(A)
The manufacturers MSDS for this product is available at: http://www.deepwaterhorizonresponse.com/posted/2931/COREXIT_9500_UsCuEg.539287.pdf
See also EPA NCP page 11: http://www.epa.gov/emergencies/docs/oil/ncp/notebook.pdf
Corexit 9500 Ingredients:
propylene glycol*
organic sulfonic acid salt*
petroleum distillates, hydrotreated light
In addition, the following chemicals may be included in the dispersants, based on the NRC 2005 publication:
"Both products contain a mixture of nonionic (48 percent) and anionic (35 percent) surfactants. The major nonionic surfactants include ethoxylated sorbitan mono- and trioleates and sorbitan monooleate; the major ionic surfactant is sodium dioctylsulfosuccinate." (pages 55-56)
The NRC publication lists the following surfactants on page 55:
sodium dioctyl sulfonosuccinate (DSS)*
Span-80 (CAS # 1338-43-8) sorbitan monooleate
Tween-80 (Cas# 9005-65-6) sorbitan monooleate, ethoxylated (E20)
* These are also present in Corexit 9527A, and so their toxicity information is discussed above.
The sorbitans are not clearly specified, as discussed under Corexit 9527A, and so health effects information is not yet provided here.
Per US EPA on June 10, 2010, polypropylene glycol butyl ether, may be present in Corexit 9500. It is discussed above under Corexit 9527A.
The US EPA provided the following table with information regarding Corexit 9500 in their publication on oil spill treatment options at:http://www.epa.gov/emergencies/content/ncp/products/corex950.htm
IX. ANALYSIS FOR HEAVY METALS, CYANIDE, & CHLORINATED HYDROCARBONS | ||||||||||||||||||||||
N/D = Not detected |
This table indicates arsenic, chromium and copper are in Corexit 9500. Copper is not generally of concern for human health, though it may have ecotoxic effects. Chromium is of concern if the form of chromium VI, but not if it is chromium III. Both copper and chromium III are trace essential nutrients for people. Arsenic and chromium were not listed in a similar table for Corexit 9527A in the same EPA publication.
Arsenic is a highly toxic heavy metal. It is present at a concentration of 160 parts per billion (stated as .16 ppm above). If direct dermal contact, inhalation, or ingestion (as with ocean spray) occurs, this metal could cause health effects, depending on the dose, specific exposure conditions and individual susceptibilities. Arsenic and chromium VI attack many of the same organs as chemicals in crude oil and dispersants. With sufficient exposure, they can damage the liver, kidneys, nervous system, reproductive system, respiratory system, skin, reproductive/urogenital system, and immune system. They can cause mutations and endocrine disruption, cancer, and can harm the developing fetus.
Combined effects of multiple chemicals on the same organ system can be far more serious if they are sufficient to overwhelm the body's ability to detoxify or otherwise defend against toxic effects. There is no safe level of exposure to mutagenic carcinogens, and even very small doses can confer some level of risk.
Hydrotreated light petroleum distillates
Hydrotreated light petroleum distillates were substituted for 2-BE in the formulation of Corexit 9500. The 2005 NRC book on dispersants described the chemical that was used as a substitute for 2-BE as follows:
"the solvent was replaced by a mixture of food-grade aliphatic hydrocarbons
(Norpar 13; n-alkanes ranging from nonane to hexadecane) in Corexit 9500"
Limited information on Norpar 13 was located in preparing this report and is provided below. Due to the scarcity of information, toxicity information is also provided on a similar chemical mixture known as kerosene. It is believed to be very similar to Norpar 13 and the hydrotreated light petroleum distillates based on a review of available scientific information,
Norpar 13
Norpar 13 It contains simple hydrocarbons ranging in chain length from nine to 16 carbon atoms. The Norpar 13 MSDS is available at:
http://ilrc.ucf.edu/documents/ILRC%2000000078/MSDS%2000000078.pdf and excerpts from that MSDS are provided below.
VENTILATION
Use only with ventilation sufficient to prevent exceeding recommended exposure limit or buildup of explosive concentrations of vapor in air. No smoking, or use of flame or other ignition sources.
RESPIRATORY PROTECTION
Use supplied-air respiratory protection in confined or enclosed spaces, if needed.
PROTECTIVE GLOVES
Use chemical-resistant gloves, if needed, to avoid prolonged or repeated skin contact.
EYE PROTECTION
Use splash goggles or face shield when eye contact may occur.
OTHER PROTECTIVE EQUIPMENT
Use chemical-resistant apron or other impervious clothing, if needed, to avoid contaminating regular clothing, which could result in prolonged or repeated skin contact.
PERSONAL HYGIENE
Minimize breathing vapor or mist. Avoid prolonged or repeated contact with skin. Remove contaminated clothing; launder or dry-clean before re-use. Remove contaminated shoes and thoroughly clean and dry before re-use. Cleanse skin thoroughly after contact, before breaks and meals, and at end of work period. Product is readily removed from skin by waterless hand cleaners followed by washing thoroughly with soap and water.
NATURE OF HAZARD AND TOXICITY INFORMATION
Prolonged or repeated skin contact with this product tends to remove skin oils, possibly leading to irritation and dermatitis; however, based on human experience and available toxicological data, this product is judged to be neither a "corrosive" nor an "irritant" by OSHA criteria.
Product contacting the eyes may cause eye irritation.
Product has a low order of acute oral and dermal toxicity, but minute amounts aspirated into the lungs during ingestion or vomiting may cause mild to severe pulmonary injury and possibly death.
___________________________________________________________________________SECTION 11: TOXICOLOGICAL INFORMATION
___________________________________________________________________________
NATURE OF HAZARD AND TOXICITY INFORMATION
Prolonged or repeated skin contact with this product tends to remove skin oils, possibly leading to irritation and dermatitis; however, based on human experience and available toxicological data, this product is judged to be neither a "corrosive" nor an "irritant" by OSHA criteria.
Product contacting the eyes may cause eye irritation.
Product has a low order of acute oral and dermal toxicity, but minute amounts aspirated into the lungs during ingestion or vomiting may cause mild to severe pulmonary injury and possibly death.
This product is judged to have an acute oral LD50 (rat) greater than 5 g/kg of body weight, and an acute dermal LD50 (rabbit) greater than 3.16 g/kg of body weight.
ECOLOGICAL INFORMATION
Do not discharge this product into public waters or waterways unless authorized by a National Pollution Discharge Elimination System (NPDES) permit issued by the Environmental Protection Agency (EPA).
Environmental and Ecological data may be available for this product. Write or call ExxonMobil to obtain further information. Refer to Section 6 and Section 15 for Accidental Release information and Regulatory Reporting information.
__________________________________________________________________________
Note - the information above is relevant to Norpar 13 alone. When Norpar 13 is used in a dispersant, it is diluted by other ingredients. The toxicity of the product depends on the combination of its ingredients and so may be more or less toxic than this ingredient.
Kerosene
A review of information on chemical and physical properties and other related characteristics for the "hydrotreated light petroleum distillates", as listed in the MSDS for Corexit 9500, the MSDS for Norpar-13, US EPA's NCP guide, and other sources linked above was carried out. Dr. Alex Madonik, a consulting chemist with the Green Science Policy Institute (http://www.greensciencepolicyinstitute.org/) evaluated the available science, considered that aromatic components (e.g., benzene) were removed by hydrotreating, and that other refinements may have been made to remove impurities. Dr. Madonik found that the hydrotreated light petroleum distillates and Norpar 13 are very similar to high grade kerosene, a fuel that has far more toxicity information than Norpar 13, including critical information on longer-term exposure effects.
Given the lack of short and long-term animal and human testing data regarding the toxicity Norpar-13 or the full dispersant, there are critical gaps in public health hazard information. Information is available on many aspects of kerosene toxicity. There are limitations to this approach, but recognizing apparent similarities with Norpar 13, the information below from a federal toxicity summary on kerosene may be a useful supplement.
The information below regarding health effects was obtained from the recent review of kerosene toxicity in the 2008 CDC Toxicological Profile, unless otherwise noted (see "sources" below) The types of toxic effects caused by short term exposure to kerosene are similar to those listed for Norpar 13 ((e.g., nervous system depression, skin irritation). If additional refinement of the chemical composition is provided by the manufacturer or US EPA and indicates that Corexit 9500 ingredients have a different chemical composition than indicated in their current website information and NRC 2005, updated information will be provided (check for updated reports on our website at www.sciencecorps.org/crudeoilhazards.htm).
The information below is provided as the best available approximation at this time, and is offered to assist the public and health professionals in considering protective strategies and potential health effects. As noted above, in the case of actual poisoning, the manufacturer is required to provide complete ingredient information to an individual's health care provider.
Kerosene is a toxic fuel with extensive information regarding acute and chronic health effects. While some animal studies specify the grade of kerosene, human poisoning incidents and other human exposure situations rarely provide an opportunity to determine the grade of kerosene with any precision. That may play a role in the relevance of the following information for Norpar 13 and the dispersant from which it is formulated.
Crude oil is an unrefined combination of simple and complex hydrocarbons. People will likely be exposed to a combination of the dispersant and crude oil. Slight variations in the long-chain hydrocarbon composition of a dispersant's ingredients may not have a noticeable impact on the toxicity of the crude oil-dispersant combination.
As with many other hydrocarbon solvents, kerosene can enter the body when it is absorbed through the skin, or through inhalation or ingestion. It is poorly absorbed in the gastrointestinal system. Kerosene can cause serious immediate respiratory health problems if substantial amounts are inhaled. In addition, inhalation or dermal exposure to kerosene can cause bronchoconstriction, which is relevant to asthmatics and other people with respiratory disorders.
Kerosene can damage the skin directly, causing blisters, erythema, peeling skin, reddening, soreness, burning, swelling, and other damage. Kerosene vapors can cause eye irritation. Dermal Exposure has caused hyperactivity and hyper-responsiveness to tactile stimulation.
Oral exposure to kerosene can cause vomiting, abdominal pain, gastroenteritis, bleeding and diarrhea. It is unlikely that people would be exposed to concentrations that could cause these effects as a result of dispersant use. However, the combination of crude oil exposure, which contains many of the same chemicals that are in kerosene, may result in high exposures under some conditions.
Due to the vomiting caused by these chemicals, aspiration can occur (inhalation of some materials from vomiting). This is of considerable concern. Crude oil, kerosene, and other dispersant chemicals are hazardous when inhaled, since they can coat the lungs and cause chemical pneumonitis.
Lower levels of exposure to kerosene in air, or through aspiration can cause asthma-like responses because it:
1) acts on the parasympathetic pathway exerting a direct effect on the vagus nerve,
or
2) inhibits acetylcholinesterase, leading to bronchoconstriction.
It may also change the ionic flow across cellular membranes, to prolong muscle contraction.
Kerosene has caused damage to the blood forming (hematopoietic)system in people, with reduced leukocyte counts and other effects. Animal studies also found abnormalities in this system following dermal exposure. There may be greater susceptibility in some people to this effect. The effect may also be due to impurities in lower grade kerosene (e.g., if benzene is present).
Tachycardia and cardiomegaly have been reported in children with high oral exposures to kerosene. Some animal studies also reported cardiovascular damage, including aortic plaques. Animal studies also found increased serum cholesterol and decreased HDL.
In longer term exposure animal studies, decreased blood glucose levels and increased levels of lactate and pyruvate were observed. These can cause metabolic acidosis.
Liver pathology has also been reported.
Neurological effects include disruption of the central nervous system in children, following ingestion of kerosene by children. A reduction in the odor and taste recognition of kerosene can occur in some people after a period of exposure. Animal studies have also observed central nervous system effects, including drowsiness, lack of muscular coordination, and behavioral changes.
CDC concluded in 2008 that :
"The information from human and animal studies indicates that neurotoxicity may occur by all routes of exposure, and that all fuel oils may be neurotoxic. As is common with hydrocarbons, the primary acute neurotoxic effect is central nervous depression that may be manifest in a number of symptoms."
(CDC, 2008, page 81, underlining added for emphasis)
Disruption of the immune system as reported in animal studies. Genotoxicity studies indicate that kerosene may cause mutations, including chromosome aberrations in bone marrow. This is similar to many other chemicals with the same type of hydrocarbon structure. It also caused mutations in many genetic test systems. There is some evidence that kerosene may cause cancer. However, it is important to recognize that the removal of aromatics (e.g., benzene) from kerosene, as specified in the Corexit ingredient listing, is likely to reduce and perhaps eliminate the capacity of this ingredient to cause mutations and cancer.
CDC (2008) indicates that people who are exposed occupationally or frequently in other situations may be at higher risk than those exposed with less frequent exposures.
Summary
Based on toxicity observed in humans and in animal studies, kerosene can damage the following systems, depending on the route and amount of exposure, and individual characteristics: respiratory, neurological, gastrointestinal, cardiovascular system, metabolic, hematopoietic, liver, skin, and may cause mutations. CDC established a minimum risk level for kerosene of 0.02 milligrams per meter cubed (mg/m3) for moderate term exposure (15 to 364 days).
Sources
CDC-ATSDR Toxicological Profile for Fuel Oils, 2008. http://www.atsdr.cdc.gov/toxprofiles/tp75-c2.pdf Kerosene is one of many fuels covered, so study specifics must be carefully reviewed.
MSDS for Norpar 13 by ExxonMobil: http://ilrc.ucf.edu/documents/ILRC%2000000078/MSDS%2000000078.pdf
The combination of a dispersants and crude oil can be more toxic than either alone, since they have many of the same toxic properties. In addition, based on the basic properties of dispersants, they may make it easier for crude oil to enter the body, enter cells, and cause damage, as noted for many dispersant ingredients above.
Multiple chemicals with the same target organ (e.g., the skin, nervous system, kidneys, reproductive system, respiratory system) can cause greater damage. They can have additive effects, or even cause the total burden on an organ system to reach a critical level. A combination of similarly acting chemicals can exceed a safety threshold, making serious health problems more likely (e.g., exceeding detoxification capacity, or other essential physiological processes).
An article on chemical mixtures by Dr. David Carpenter, Director of the Environmental Institute at the State University of New York in Albany discusses this problem. Additional sources of information are listed in Dr. Carpenter's article. In addition, the US EPA provides publications on chemical mixtures, and requires consideration of mixtures in situations where they are often encountered (e.g., hazardous waste sites). This information can be obtained through a search of their website at: www.epa.gov
The combination of crude oil chemicals and dispersants can cause health effects in people and wildlife, depending on the amount of exposure they have, and their susceptibility to the mixture of chemicals that they are exposed to.
Information is needed on chemical concentrations in air, water, seafood and other media. This information has not been adequately provided as discussed below.
Crude oil is toxic and its chemical ingredients can damage every system in the body. Dispersant chemicals can also affect many of the same organs. This can increase the risk and severity of harm.
The systems in the body that are known to be affected by these chemicals include:
respiratory system nervous system, including the brain
liver reproductive/urogenital system
kidneys endocrine system
circulatory system gastrointestinal system
immune system sensory systems
musculoskeletal system hematopoietic system (blood forming)
skin and integumentary system disruption of normal metabolism
Damage to these systems causes a wide range of diseases and conditions. In addition, crude oil chemicals can interfere with normal growth and development through endocrine disruption and direct damage to fetal tissue (CDC, 1999). Chemicals in crude oil can also cause DNA damage, cancer and multi-generational birth defects.
Dispersants used in the Gulf can increase the uptake of crude oil's chemical ingredients into the body, and can increase their movement into critical organs. The dispersant being used contains many ingredients that target the same organs in the body as crude oil. The combination of toxic effects from crude oil and dispersants may pose greater public health risks when exposure to the combination occurs.
Especially susceptible people include:
- those with pre-existing health conditions
- infants, children, and unborn babies
- people working or living under conditions that impose health stresses
- pregnant women, especially those carrying more than one baby
Individual responses to exposure depend on how or one or more chemicals enter their body and their individual characteristics and susceptibilities.
Failure to Warn and Failure to Fully Disclose Test Results
Chemicals in crude oil and dispersants can break down, interact, and change form as they move through salt water, are subjected to sunlight, interact with each other and with the chemicals in the air. The best way to determine the composition of chemicals that reach the shore, workers on boats, or become incorporated into the food supply is to take samples of the air, water, and food and have it tested.
The US EPA Gulf spill sampling website at http://www.epa.gov/bpspill/sediment.html contains the following text:
"EPA has observed odor-causing pollutants associated with petroleum products along the coastline at low levels. Some of these chemicals may cause short-lived effects like headache, eye, nose and throat irritation, or nausea. People may be able to smell some of these chemicals at levels well below those that would cause short-term health problems."
It is a very serious oversight that they don't provide any warning that children and adults with asthma and other respiratory health problems (e.g., COPD) can experience serious health problems as a result of exposure to irritants, including those from oil spill chemicals.
The clearly do not warn people that many volatile organic chemicals from crude oil can have serious long term health consequences, including cancer and that some can cause birth defects and mutations.
The report does not describe the chemicals that people are smelling so that the public health community and the public have an opportunity to evaluate whether the chemicals involved are safe.
Although US EPA has listed very extensive sampling and analysis plans on their spill website, they have not provided most of the results that they have.
Their water data link is at: http://www.epa.gov/bpspill/water.html#data It has highlighted text for additional information. Many of these links still do not work.
Although the EPA webpage provides links that appear to go to their air sampling data, most links to air results are non-functional or do not provide useful information. An example of this can be seen at: http://www.epa.gov/bpspill/air.html#datarep
An image from that website is shown below.
When you click on "view" for any location, this is what the results look like:
MIME-Version: 1.0
Content-Type: Multipart/Related;
type="text/html";
boundary="saw_part_boundary_0"
--saw_part_boundary_0
Content-Type: text/html; charset=utf-8
Content-Transfer-Encoding: base64
PCFET0NUWVBFIEhUTUwgUFVCTElDICItLy9XM0MvL0RURCBIVE1MIDQuMC8vRU4iPg0KPGh0bWwg
ZGlyPSJsdHIiIHhtbG5zPSJodHRwOi8vd3d3LnczLm9yZy8xOTk5L3hodG1sIiB4bWw6bGFuZz0i
ZW4iIGxhbmc9ImVuIj4NCjxoZWFkPg0KPG1ldGEgaHR0cC1lcXVpdj0iQ29udGVudC1UeXBlIiBj
b250ZW50PSJ0ZXh0L2h0bWw7IGNoYXJzZXQ9dXRmLTgiPg0KPG1ldGEgaHR0cC1lcXVpdj0iQ29u
dGVudC1MYW5ndWFnZSIgY29udGVudD0iZW4iPg0KPHRpdGxlPk9yYWNsZSBCSSBJbnRlcmFjdGl2
ZSBEYXNoYm9hcmRzPC90aXRsZT4NCjxsaW5rIGhyZWY9ImNpZDozOWRiMzRmMSIgdHlwZT0idGV4
dC9jc3MiIHJlbD0ic3R5bGVzaGVldCI+PC9saW5rPg0KPGxpbmsgaHJlZj0iY2lkOjM5ZGIzNGYy
Source: http://www.epa.gov/bpspill/reports/mht/c02_pm.mht copied June 8, 2010
This type of information was retrieved over many weeks of attempting to review sampling results, using multiple computer systems. The failure of the federal government to provide the full range of sampling results in readily accessible form deprives workers, the general public, and health care providers is a serious problem. It deprives them of essential information about what people have been and are likely to be exposed to in communities and at work. Some information is provided (e.g., BTEX chemicals), but many important details are lacking, and the chemical list is insufficient.
As with the other information gaps, the lack of information prevents full evaluation of potential health risks. Even more important in protection of public health, we are unable to identify those at greatest risk or recommend the best protective strategies that can reduce disease and disability. A list of essential information was submitted to Congressional representatives who were holding hearings on dispersants, as described below
US EPA has reported high levels of hydrogen sulfide and volatile organic chemicals (VOCs) were present in the air on shore in Louisiana. These may be due to crude oil alone, or in combination with dispersants. Hydrogen sulfide has an initial odor, but also has a unique capacity to cause acclimation to the smell. That is, people become accustomed to it quickly and will not recognize they are in an area with high levels of hydrogen sulfide. That has caused substantial illness and even death in some cases.
Hydrogen sulfide is also an irritant that poses serious health risks for people with respiratory disorders, including those with asthma and COPD.
VOCs include hundreds of chemicals, many very toxic. Benzene, a human carcinogen, was discussed above. Other VOCs in crude oil as well as chemicals in dispersants are of concern.
It is important that the federal government and state agencies provide the results of their air, water, seafood, and other testing to the public as soon as the information becomes available. The public and their health care providers can only make informed choices when they have sufficient information regarding the chemicals present in their homes, workplaces, and communities.
Communicating with Congress
Many organizations and some members of Congress have asked the federal government to require disclosure of chemical spill information that is essential in order to fully protect public health and treat those who have become ill while working on the spill.
Complete chemical information is essential in order to:
- identify potential health effects
- warn workers and the public about hazards
- identify those people who are at high risk
- recommend appropriate protective actions, clothing, equipment, & strategies
The request below was provided to Congress in May 2010 in preparation for hearings on the Gulf spill.
"The following questions should be posed to the companies and federal agencies involved in the Gulf spill and its cleanup:
1) What products (detergents, surfactants, etc) have been used on the spill in addition to Corexit 9500 and 9627?
2) What are the chemical ingredients in these products (including Corexits)? CBI is not relevant when people's health is at stake.
3) When will the full range of scientific data regarding the products been disclosed?
EPA would normally require:
- studies showing of persistence and bioaccumulation potentials, with anticipated half-lives in media, marine life, and mammals
- a complete list of breakdown products that occur in the environment, including what is expected in marine life and mammals (i.e., humans)
- a complete list of likely reactive byproducts, including those produced in a marine environment, produced through interaction with crude oil and sun, and through other likely interactions (e.g., attempted burns of surface crude oil)
- the persistence and bioaccumulation potentials of the breakdown and reactive byproducts, which can create dozens of new chemicals
4) When will the full toxicity data on all of the above be posted on a website? This should include studies submitted by the manufacturers to EPA, NOT useless summaries.
5) Why isn't there a federal site that provides adverse effects information from the numerous previous uses of these products? This should cover wildlife, environmental media, and human populations. This information was collected after Corexit 9527 was used in Alaska.
6) Why have only the companies very brief and relatively useless summaries (e.g., material safety data sheets) been posted by EPA? These are company files, and are not evaluations by scientists who have no conflict of interest.
7) Why has the extensive monitoring data that EPA has collected that could tell us what chemicals are in the air and water been kept secret? Why has only limited summary information been provided to the public?
Why do we need this information?
In past disasters, inadequate public information and protections have caused serious health problems among responders and local communities that were poorly informed about hazards. Long after 9/11, cleanup workers remained uninformed and unprotected as they were exposed to carcinogens, neurotoxins, and other hazards. Negligent actions of many agencies led to thousands of severely harmed workers who are disabled and often dying. This occurred without any good reason. Adequate information and protective equipment could have protected those who worked after the initial attack.
The human toll of the Gulf oil spill will rise far above the eleven men who were initially killed when the oil rig exploded unless the current federal pattern of secrecy and inadequate protections changes. There is no excuse for failing to inform and protect workers and the public, at a minimum offering them accurate information and recommending options for protection.
Federal, state, and local agencies' desire to keep this information secret is unjustified and dangerous. There is no place for politics or selfish personal interests when people are exposed to chemicals that can cause disease and death. Current actions reflect the opposite of protective public health strategies. Those who place the public interest and well being above corporate and agency interests must be allowed to take appropriate actions in this situation to insure the best possible current and future health for people in the Gulf Region.
Any assistance you can provide in obtaining the answers to the questions above, and insuring competent federal staff are working on the Gulf spill would be appreciated."
Regards,
Michael R. Harbut, MD
Wayne State University
Professor, Clinical Medicine
Director, Environmental Cancer Initiative
Director, Environmental Cancer Initiative
Chief, Center for Occupational & Environmental Medicine
Karmanos Cancer Institute,
118 N. Washington,
Detroit, MI 48067-1751
Kathleen Burns, Ph.D.
Director
Sciencecorps
Lexington, MA, USA
On June 8, 2010 EPA disclosed Corexit ingredients on their dispersant website: http://www.epa.gov/bpspill/dispersants.html
They did not provide the extensive information that they have from testing air, water, soil and sand, and ecological materials for chemical contamination. They did not disclose studies showing chemical breakdown products in an ocean environment, chemicals produced by interaction with crude oil, or other technical information necessary to fully assess potential human and ecological effects (as identified in the listed submitted to Congress above).
The following information is shown as provided by US EPA at: http://www.epa.gov/bpspill/dispersants.html
.
CAS Registry Number | Chemical Name |
57-55-6 | 1,2-Propanediol |
111-76-2 | Ethanol, 2-butoxy- |
577-11-7 | Butanedioic acid, 2-sulfo-, 1,4-bis(2-ethylhexyl) ester, sodium salt (1:1) |
1338-43-8 | Sorbitan, mono-(9Z)-9-octadecenoate |
9005-65-6 | Sorbitan, mono-(9Z)-9-octadecenoate, poly(oxy-1,2-ethanediyl) derivs. |
9005-70-3 | Sorbitan, tri-(9Z)-9-octadecenoate, poly(oxy-1,2-ethanediyl) derivs |
29911-28-2 | 2-Propanol, 1-(2-butoxy-1-methylethoxy)- |
64742-47-8 | Distillates (petroleum), hydrotreated light |
Dispersant Effects
What are the tradeoff considerations being weighed regarding the impact of fish and wildlife when making decisions about the subsea use of dispersants?
Dispersants are generally less toxic than oil. When considering the use of a dispersant in the deep ocean, the federal government weighs the effectiveness of the dispersant in breaking down the oil at such depths, the benefits of preventing the oil from rising to the surface and eventually hitting the shore where it is likely to do significant damage to birds, wetlands and aquatic life, and the long term impacts of the dispersant mixed with oil in deeper waters. We have a monitoring and sampling plan in place to track the movement of the oil and we reserve the right to stop the use of these dispersants at any time based on the results.
Dispersants are generally less toxic than oil. When considering the use of a dispersant in the deep ocean, the federal government weighs the effectiveness of the dispersant in breaking down the oil at such depths, the benefits of preventing the oil from rising to the surface and eventually hitting the shore where it is likely to do significant damage to birds, wetlands and aquatic life, and the long term impacts of the dispersant mixed with oil in deeper waters. We have a monitoring and sampling plan in place to track the movement of the oil and we reserve the right to stop the use of these dispersants at any time based on the results.
Are any human health effects expected as a result of using the dispersants?
People working with dispersants are strongly advised to use a half face filter mask or an air-supplied breathing apparatus to protect their noses, throats, and lungs, and they should wear nitrile or PVC gloves, coveralls, boots, and chemical splash goggles to keep dispersants off skin and out of their eyes. CDC provides more information on reducing occupational exposures while working with dispersants during the Gulf Oil Spill Response.
People working with dispersants are strongly advised to use a half face filter mask or an air-supplied breathing apparatus to protect their noses, throats, and lungs, and they should wear nitrile or PVC gloves, coveralls, boots, and chemical splash goggles to keep dispersants off skin and out of their eyes. CDC provides more information on reducing occupational exposures while working with dispersants during the Gulf Oil Spill Response.
Material Data Safety Sheet for Corexit 9500A (PDF) (11pp., 88 K, About PDF)
Material Data Safety Sheet for Corexit 9527A (PDF) (11 pp., 132 K, About PDF)
What effects could the use of dispersants have on marine life?
It’s important to understand that the use of dispersants is an environmental trade-off. We know dispersants are generally less toxic than the oils they breakdown. We know that surface use of dispersants decreases the environmental risks to shorelines and organisms at the surface and when used this way, dispersants breakdown over several days. However the long term effects on aquatic life are unknown, which is why EPA and the Coast Guard are requiring BP to implement a robust sampling and monitoring plan.
It’s important to understand that the use of dispersants is an environmental trade-off. We know dispersants are generally less toxic than the oils they breakdown. We know that surface use of dispersants decreases the environmental risks to shorelines and organisms at the surface and when used this way, dispersants breakdown over several days. However the long term effects on aquatic life are unknown, which is why EPA and the Coast Guard are requiring BP to implement a robust sampling and monitoring plan.
The federal response is intended to ensure that these operations are constantly monitored for any short or long term adverse effects that may outweigh the benefit of using dispersants.
"Derivitives"
Note - the term "derivs" in the table above means "derivitives". That indicates there are various other chemicals similar to the one that is listed. It is unclear what these other chemicals are, other than the fact that they are related to the ones listed.
Connecting EPA's Dispersant Ingredient List to Common Chemicals.
Most chemicals have multiple chemical names and many also have common names that are used under different circumstances. The following information connects the chemical names commonly found in the medical science literature and used in this report, to the names that were used by US EPA in describing dispersant ingredients in the table above. The extensive list of possible chemical names for a chemical is illustrated by the last entry below.
CAS # Name on EPA's list and the name we have used in the webpage text
57-55-6 1,2-Propanediol = propylene glycol
111-76-2 Ethanol, 2-butoxy = 2-BE
577-11-7 Butanedioic acid, 2-sulfo-, 1,4-bis(2-ethylhexyl) ester, sodium salt (1:1)
= DSS
64742-47-8 Distillates (petroleum), hydrotreated light
= similar to kerosene and Norpar 13
The chemical composition of the sorbitans listed below have not been fully described and so are not discussed in this report.
1338-43-8 Sorbitan, mono-(9Z)-9-octadecenoate
9005-65-6 Sorbitan, mono-(9Z)-9-octadecenoate, poly(oxy-1,2-ethanediyl) derivs
9005-70-3 Sorbitan, tri-(9Z)-9-octadecenoate, poly(oxy-1,2-ethanediyl) derivs
Newly disclosed ingredient without information on which product contains the chemical or the percentage of it comprises in the product:
29911-28-2 2-Propanol, 1-(2-butoxy-1-methylethoxy) = see Chemical Synonyms below.
Chemical Synonyms
2-Propanol, 1-(2-butoxy-1-methylethoxy) is a product of questionable importance in the Corexit products, since it is not listed on the MSDS sheets, and it is unclear how much is contained in the products. It has a number of synonyms and many "related" chemicals in the propylene glycol ether family. To fully capture all relevant toxicity reports, these terms require searching.
The list of chemical synonyms below was derived from multiple sources, including the links listed below. The first link, to the National Library of Medicine's website, includes the example of this chemical's information.
Links:
Synonyms and Related Chemicals
1-(2-Butoxy-1-Methylethoxy)-2-Propanol
1-(2-Butoxy-1-methylethoxy) propan-2-ol
2-Propanol, 1-(2-butoxy-1-methylethoxy)-
Ambiflo L-317
Butoxy polypropylene glycol
Butoxypolypropylene glycol 400 & 800
Butoxypropanediol polymer
Butyl dipropasol solvent
Crag fly repellent
Dipropylene glycol butoxy ether
Dipropylene glycol monobutyl ether
Dipropylene glycol butyl ether
Dowanol 54B
EINECS 249-951-5
ENT 8286
Exp. miticide No. 7
Fluid-AP
Newpol LB3000
OPSB
Poly(oxy(methyl-1,2-ethanediyl)) , alpha-butyl-omega-hydroxy-
Poly(oxypropylene) butyl ether
Poly(propylene oxide) , monobutyl ether
Polyoxypropylene (all the following: 4, 5, 9, 12, 14, 18, 22, 24, 26, 30, 33, 40, 52, 54)
Polyoxypropylene glycol butyl monoether
Polyoxypropylene monobutyl ether
Polyoxypropylene(40) butyl ether
Polypropylene glycol #400, monobutyl ether
Polypropylene glycol (all: 2, 4, 9, 12, 14, 15, 16, 17, 20, 22, 24, 26, 30, 33, 40, 52, 53) butyl ether
Polypropylene glycol butyl ether
Polypropylene glycol monobutyl ether
Polypropyleneglycol #800, monobutyl ether
PPG - (all: 2, 4, 5, 9, 12, 14, 15, 16, 17, 18, 20, 22, 24, 26, 30, 40, 52, 53) - butyl ether
Stabilene
Stabilene fly repellent
Ucon fluid LB-3000
Ucon LB all of the following: 1145, 1800X, 1145, 250, 3000 (fluid), 525, 625, 65
Ucon Lubricant LB-1145, 3000
Unilube MB-370
We gratefully acknowledge the assistance of the following people in the preparation of this report and with previous work that contributed to this report: Richard Charter, Alex Madonik, Peter Burns, Kenneth Rudo, John Cunningham, Charlotte Wells, and the City of San Francisco's environmental department for sponsoring work on 2-BE cited in this report. The acknowledgement of these parties does not indicate their agreement with the full contents of the report.
Authors
Kathleen Burns, Ph.D. Director, Sciencecorps
Lexington, Massachusetts
Dr. Burns worked for state and federal agencies for 25 years before founding Sciencecorps in 2004. She has published books and papers on the toxicology and epidemiology of chemicals, radiation, and nanomaterials. She managed investigative teams and conducted risk assessments of air, water, soil, and food contamination in the US and other countries, and for 9/11 responders, Veterans, tribal organizations, and post-Katrina communities. She has worked on improved air and water regulations, fish advisory programs, TSCA regulations, community right-to-know protections, and other state and federal programs. Dr. Burns has degrees from the University of
Chicago and the University of Illinois Medical Center in Chicago.
Michael R. Harbut, M.D.
Chief, Center for Occupational & Environmental Medicine;
Director, Environmental Cancer Initiative, Karmanos Cancer Institute
118 N. Washington, Royal Oak, Michigan 48067-1751 248.547.9100 e-mail: harbutm@karmanos.org
118 N. Washington, Royal Oak, Michigan 48067-1751 248.547.9100 e-mail: harbutm@karmanos.org
Michael R. Harbut, MD, MPH is Clinical Professor of Internal Medicine and Director of the Environmental Cancer Program at Wayne State University's Karmanos Cancer Institute. Board Certified in Occupational and Environmental Medicine, Harbut was Chair of the Occupational and Environmental Health Section of the American College of Chest Physicians, was Medical Coordinator of the Kibumbe Refugee Camp during the 1994 Civil War in Rwanda where the death rate for patients under his care was 1/3 that of the remainder of the camp and was Chief US Medical Advisor to Poland's Solidarity during the Cold War. His research has been published or presented in venues ranging from the New England Journal of Medicine to the White House.
Copyright Sciencecorps, Lexington MA, June 2010
All rights reserved.

After the last river has been poisoned
Only after the last fish has been caught
Then you will find that money cannot be eaten.
-- Cree prophecy --



